Classification Of Matter
ATOMS
The atom is the basic
building block for all matter in the universe. Atoms are extremely small and
are made up of a few even smaller particles. The basic particles that make up
an atom are electrons, protons, and neutrons. Atoms fit together with other atoms
to make up matter. It takes a lot of atoms to make up anything. There are so
many atoms in a single human body we won't even try to write the number here.
Suffice it to say that the number is trillions and trillions (and then some
more).
There are different
kinds of atoms based on the number of electrons, protons, and neutrons each
atom contains. Each different kind of atom makes up an element. There are 92
natural elements and up to 118 when you count in man-made elements.
Atoms last a long time,
in most cases forever. They can change and undergo chemical reactions, sharing
electrons with other atoms. But the nucleus is very hard to split, meaning most
atoms are around for a long time.
Structure
of the Atom
At the center of the
atom is the nucleus. The nucleus is made up of the protons and neutrons. The
electrons spin in orbits around the outside of the nucleus.
The
Proton
The proton is a
positively charged particle that is located at the center of the atom in the
nucleus. The hydrogen atom is unique in that it only has a single proton and no
neutron in its nucleus.
The
Electron
The electron is a
negatively charged particle that spins around the outside of the nucleus.
Electrons spin so fast around the nucleus, scientists can never be 100% sure where
they are located, but scientists can make estimates of where electrons should
be. If there are the same number of electrons and protons in an atom, then the
atom is said to have a neutral charge.
Electrons are attracted to the nucleus by the
positive charge of the protons. Electrons are much smaller than neutrons and
protons. About 1800 times smaller!
The
Neutron
The neutron doesn't
have any charge. The number of neutrons affects the mass and the radioactivity
of the atom.
ELEMENTS
An element is a pure
substance that is made from a single type of atom. Elements are the building
blocks for all the rest of the matter in the world. Examples of elements
include iron, oxygen, hydrogen, gold, and helium.
Atomic Number
An important number in an element is the atomic
number. This is the number of protons in each atom. Each element has a unique
atomic number. Hydrogen is the first element and has one proton, so it has an
atomic number of 1. Gold has 79 protons in each atom and has an atomic number
of 79. Elements in their standard state also have the same number of electrons
as protons.
Silicon
(Atomic number 14) is an important element in electronics
Forms
of an Element
Even though elements
are all made from the same type of atoms, they can still come in different
forms. Depending on their temperature they can be solid, liquid, or gas. They
can also take different forms depending on how tightly the atoms are packed
together. Scientists call these allotropes. One example of this is carbon.
Depending on how carbon atoms fit together they can form diamond, coal, or
graphite.
How
many elements are there?
There are currently 118
known elements. Of these, only 94 are thought to naturally exist on Earth.
Families
of Elements
Elements are sometimes
grouped together because they have similar properties. Here a few of the types:
Noble
Gases - Helium, neon, argon, krypton, xenon, and radon
are all noble gases. They are unique in that the outer shell of their atoms is
full of electrons. This means they don't react much with other elements. They
are often used in signs as they glow in bright colors when an electrical
current is passed through them.
Alkali
Metals - These elements have just 1 electron in the outer
shell of their atom and are very reactive. Some examples are lithium, sodium,
and potassium.
Other groups include transition metals, nonmetals,
halogens, alkali earth metals, actinides, and lanthanides.
Periodic
Table
An important way of
learning and understanding elements for chemistry is the periodic table. You
can learn more about this on our periodic table of elements page.
Fun
Facts about Elements
·
Elements found on Earth and Mars are
exactly the same.
·
Hydrogen is the most common element
found in the universe.
·
It is also the lightest element.
·
Isotopes are atoms of the same element,
with different numbers of neutrons.
·
In ancient times the elements referred
to fire, earth, water, and air.
·
Helium is the second most common element
in the universe, but is very rare on the Earth.
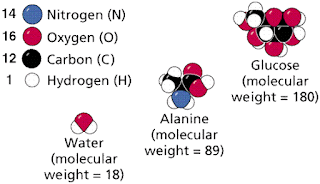
MOLECULES
Molecules are compounds in which
the elements are in definite, fixed ratios, as seen in figure below. Those
atoms are held together usually by one of the three types of chemical bonds
discussed above. For example: water, glucose, ATP. Mixtures are compounds with
variable formulas/ratios of their components. For example: soil. Molecular
formulas are an expression in the simplest whole-number terms of the
composition of a substance. For example, the sugar glucose has
6 Carbons, 12 hydrogens, and 6 oxygens per repeating structural unit. The
formula is written C6H12O6.
. Determination of molecular weights by addition of the weights of the
atoms that make up the molecule.
|
COMPOUNDS
Microscopic view of the molecules of the compound
water (gas phase). Oxygen atoms are red and hydrogen atoms are white.
Note that a compound:
|





give me and explanation and example of elements beloging to metaloid element and a liquid element at room temperature?
BalasHapuslet metalloid element is a chemical element that has a group of properties between metals and non-metals. Metalloid difficult to distinguish from metal, the main difference is that generally metalloids are semiconductors, while the metal is a conductor. There are eight elements that are classified as metalloids, namely boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po), and astatine (At).
HapusMost metal elements are elements that we know among the 108 elements.
conotoh of metalloid elements, namely:
- Silicon is a chemical element in the periodic table that has the symbol Si and atomic number 14. It is the second most common element on earth. Is paramagnetic compounds formed. This chemical element was discovered by Jöns Jakob Berzelius.
Here there are two kinds of metal that look at room temperature elements:
The nature of the metal element:
1. At room temperature it is a solid, except for mercury (mercury) cesium, francium, and gallium liquid.
Non Metallic Elements possess the following properties:
1. At room temperature, there are solid, liquid, gaseous and in general.
hopefully understandable yes
hi ayu azura , i want ask you abaout formulas . try to mention some formula from a homogeneous mixture ?
BalasHapushay also fira. Okay I will answer the question. Here homogeneous mixture, namely: the homogenization of a mixture of differentiated between solute and solvent. example: sugar water, salt, syrup, etc. Also referred to as a homogeneous mix solution. Homogeneous is Latin for "the same kind".
HapusHomogenous mixture is a mixture in which there is the composition and properties of the same substance. Here you ask the right formula of homogeneous, well then I will give you the formula is water: H2O, ammonia: NH3, vinegar: CO3COOH, sucrose (sugar): C12H22O11. hopefully understood, thanks
Komentar ini telah dihapus oleh pengarang.
BalasHapusThe difference is that protons are positively charged atoms while neutrons are neutral atoms.
HapusThe equation, neither protons nor neutrons is motionless.
Komentar ini telah dihapus oleh pengarang.
BalasHapusMolecules are formed when two or more atoms join together chemically. While the compound is a molecule containing at least two different elements. All compounds are molecules but not all molecules are compounds.
Hapuswhy every atom have a diffrent atomic number?
BalasHapusBecause atoms have different numbers of protons and electrons
HapusHow do we determine the atomic number?
BalasHapusThe atomic number of an element is the number of protons in the nucleus of a single atom of that element.
HapusWhy each elements has a different form?
BalasHapusIs there an atom that is composed of only protons or neutrons?
BalasHapus